Data Logger for Pharma and Healthy
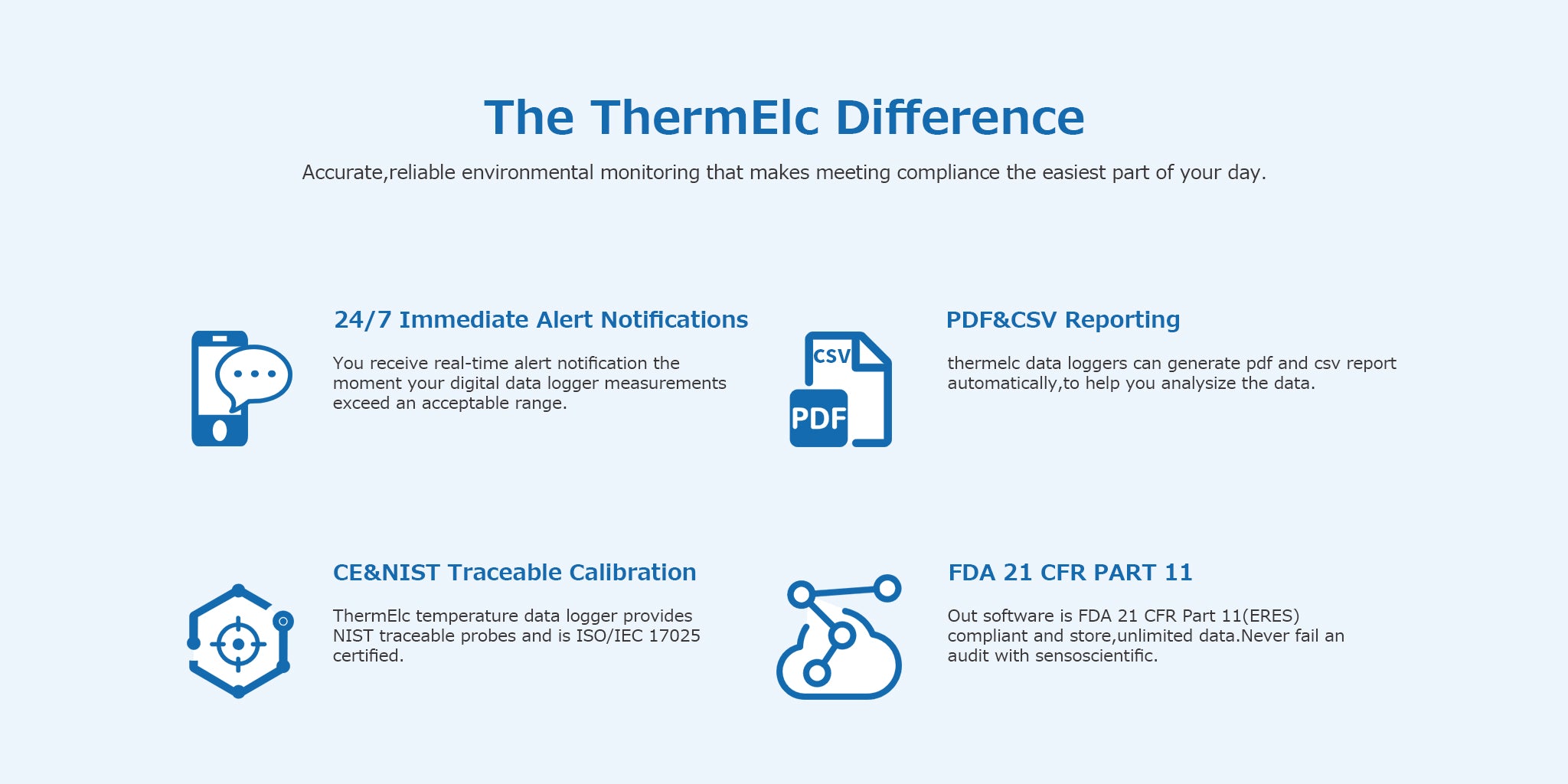
 Customers expect the highest quality of product, but if your business is in the pharmaceutical industry, it is crucial to maintain consistent quality. If you cannot prove compliance, regulatory agencies may require disposal of all drugs and vaccines. Monitoring basic data from R&D to production is crucial in protecting your assets, profits and customers. Continuous remote monitoring gives you confidence in proving compliance. Regulatory compliant temperature monitoring ensures drug quality, maximizing its shelf life and expected efficacy. Real-time alerts and deviation notifications prevent product loss and allow for immediate response.
Customers expect the highest quality of product, but if your business is in the pharmaceutical industry, it is crucial to maintain consistent quality. If you cannot prove compliance, regulatory agencies may require disposal of all drugs and vaccines. Monitoring basic data from R&D to production is crucial in protecting your assets, profits and customers. Continuous remote monitoring gives you confidence in proving compliance. Regulatory compliant temperature monitoring ensures drug quality, maximizing its shelf life and expected efficacy. Real-time alerts and deviation notifications prevent product loss and allow for immediate response.
In research, production, or application of sensitive drugs, safety is the most important factor. ThermElc's measuring instruments and integrated solutions for pharmaceuticals and healthcare ensure the quality of medical products and regulatory compliance.
Strict regulations and directives applicable throughout the manufacturing process include:
• ISO 9001 • UKCA • CE • ROHS • FCC• cGxP, cGMP (Current Good Manufacturing Practices), GLP (Good Laboratory Practices), GDP (Good Documentation Practices), etc. • 21 CFR Part 11, Electronic Records/Electronic Signatures